The Brazilian government has published the long-awaited regulation of the Clinical Research Act. Decree #12,651/2025 establishes the National System of Ethics in Research Involving Human Subjects (SINEP) and seeks to strike a balance between ethical protection for research participants and regulatory efficiency, representing the most significant regulatory milestone for Brazil’s clinical research landscape in decades.
This two-part analysis reviews the Decree’s updates, market expectations, and outstanding regulatory gaps.
1. A Scenario Long Awaited since the 2024 Legal Framework
The Clinical Research Act (Statute #14,874/2024), enacted in May 2024, was designed to confer greater legal certainty on research involving human subjects in Brazil.Previously, the subject was governed by infra-legal instruments, and the ethical review process was widely regarded as inefficient and slow.
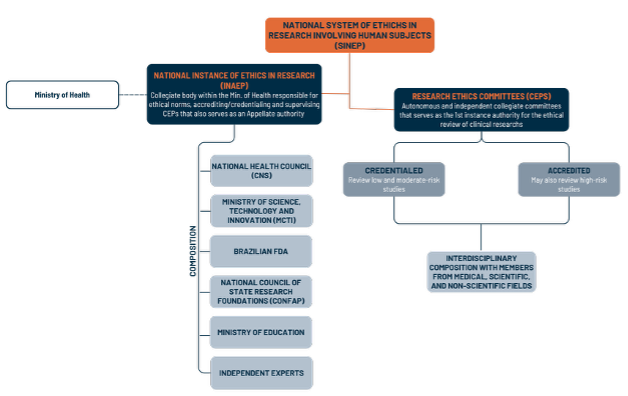
The Act’s first major innovation was the creation of a new ethics-review architecture. The National System of Ethics in Research (SINEP) is divided into:
• the National Instance of Ethics in Research (INAEP), an interdisciplinary and independent collegiate body within the Ministry of Health, entrusted with consultative, deliberative, and educational functions, with the addition responsibilities of credentialing, accrediting and serving as the Appellate instance for Research Ethics Committees (CEPs) decisions; and
• the Ethical Review Instance, represented by Research Ethics Committees (CEPs), which review clinical research protocols according to the level of risk involved.
The organizational chart below summarizes the new ethics-review architecture
However, despite notable advances, the sector had been awaiting the regulation of approximately 30 provisions of the Act. The delay risked eroding the momentum for reform, perpetuating legal and operational uncertainty. Among the key provisions requiring regulatory detail were:
• Further establishment and composition of SINEP, including INAEP and the CEPs tiering.
• Risk classification, which determines whether a protocol must be reviewed by an accredited CEP (for high-risk studies) or may be reviewed by a credentialed, non-accredited CEP (for low- or moderate-risk studies). This classification is crucial to ensure procedural proportionality and efficiency in the ethical review process;
• Post-trial access, which has been particularly controversial, in part because many considered the operational guidance insufficient to balance participant safeguards and sponsors’ financial predictability
• Mechanisms of transparency and public access to information, which remained fragile even after the enactment of the Clinical Research Act. The existing searching tools continue to present inconsistencies and limitations, hindering public access to key study information, such as the molecule under investigation, study objectives, design, eligibility criteria, and primary endpoints. Modernization of these tools is therefore essential to enhance transparency and public oversight, while safeguarding confidential and IP-sensitive data.
2. Analysis of Decree #12,651/2025: What the Decree Effectively Addresses
Institutional Structure and Ethical Governance. The Decree defines SINEP’s purpose, directing it to optimize and simplify processes, ensure Good Clinical Practice (GCP) compliance and participant protection, promote ethical/safe/efficient trials, and strengthen oversight and governance, with transparency, data protection, and integrity of research records.
Within SINEP, the Decree establishes a plural INAEP composition, as includes representatives of the National Health Council (CNS), Anvisa (Brazilian FDA), the Ministry of Science, Technology and Innovation (MCTI), the Ministry of Education (MEC), the National Council of State Research Foundations (CONFAP), and independent experts selected through a public call.
It also authorizes permanent or temporary advisory chambers to foster dialogue among regulators, the scientific community, civil society, and the private sector, and allows technical working groups with recognized experts to support credentialing, accreditation, and supervision.
The possibility of advisory chambers, and the creation of working groups with civil society participation directly addresses prior criticisms of limited representativeness, ensuring regional diversity and interdisciplinarity in ethical governance.
Credentialed and Accredited Research Ethics Committees (CEPs).
Consistent with the Clinical Research Act, the Decree differentiates credentialed and accredited CEPs. While credentialed CEPs are authorized by INAEP to review low or moderate-risk research protocols, accredited CEPs are those that have demonstrated technical and operational capacity to review high-risk protocols, in addition to lower-risk studies.
Both credentialing and accreditation will be further detailed by INAEP. Even so, the Decree already makes clear the principal distinction: accreditation is preceded by a technical assessment that includes remote or on-site inspections in order to verify compliance with ethical and technical standards.
Criteria for Risk classification. Although the precise thresholds for what constitutes a low, moderate, or high-risk study will still be set by INAEP, the Decree already specifies the criteria to be applied in risk classification. These include, for instance, whether the study includes the participation of vulnerable populations; as well as the use of emerging technologies, sensitive data, or AI; and the complexity of the study design.
The grading of risk also determines the procedural track for ethical approval: protocols will follow differentiated pathways according to their risk level, and simplified procedures may be adopted for submission, review, and authorization where appropriate.
Ethical Review by CEPs. The Decree establishes principles to guide the ethical analysis of research protocols by CEPs. The principles include the protection of dignity/safety/well-being of the participants; independence, transparency, and publicityof procedures and decisions, as well as efficiency and quality in issuing ethical opinion and the interdisciplinary and plural composition as a qualifying element of ethical judgment.
Efficiency and Predictability. The Decree operationalizes binding review timeframes: 30 business days for CEPs ethical review; 90 business days for Anvisa’s regulatory review; and 15 business days for studies deemed of strategic interest to the Unified Health System (SUS) or in response to public health emergencies.
Research of strategic relevance to the SUS are those aligned with SUS priorities, i.e. those targeting, for instance, rare diseases, pediatric populations without available therapies, domestically manufactured APIs and Partnerships for Productive Development (PDPs)/ Local Development and Innovation Program (PDIL).
Para estudos multicêntricos, o cenário também mudou. A single CEP, preferably at the coordinating center, issues one nationally valid ethical opinion and notifies the other centers, eliminating redundant reviews.
These provisions, combined with the possibility of an integrated ethical and sanitary review procedure between INAEP and Anvisa, within the scope of their respective competencies, directly address the chronic bureaucratic delays and regulatory fragmentation previously identified as major disincentives to clinical research in Brazil.
Conclusion
Decree #12,651/2025 moves Brazil from promise to execution. By standing up SINEP/INAEP, imposing binding timelines, enabling single-CEP review for multicenter trials, and opening a channel for INAEP-ANVISA integrated procedures, the government has replaced a patchwork of practices with a coherent architecture that can shorten cycle times and reduce legal uncertainty. Whether this becomes a genuine competitive edge, however, will depend on what happens next.
The government’s own note sets ambitious markers: double the number of clinical studies from the 2024 baseline (254) and catalyze an investment cycle that could more than triple, while strengthening participant protections. However, what will decide the outcome are INAEP’s Rules and operational delivery.
Part 2 of this article will analyze in depth the post-trial care regime, new transparency mechanisms, transitional provisions and next steps, including what to expect in ADI 7875- which challenges key provisions of the Clinical Research Act - and the status of the recent public consultation on clinical research (CP #69/2025), which gathered broad stakeholder input on Brazil’s clinical-research environment.
• What changed: ANVISA. Analysis of Decree #12,651/2025: What the Decree Effectively Addresses Institutional Structure and Ethical Governance .• Executive impact: Implication for executives: reassess competition & regulatory strategy posture, especially decision ownership and evidence standards across Brazil operations. .• Where to go deep: approvals, registrations, and submission workflows, data mapping, vendors, and cross‑border transfers. Context: Decree #12,651/2025 establishes the National System of Ethics in Research Involving Human Subjects (SINEP) and seeks to strike a balance between ethical protection for research participants and regulatory efficiency,. • Decisions to make: timeline to implement vs transition window, risk quantification for board reporting, internal standard + proof/evidence model.• Next steps: run a focused gap assessment; update playbooks/policies; and circulate a 1‑page executive memo (options, tradeoffs, timeline, residual risk, owner).

This section gives quick answers to the most common questions about this insight. What changed, why it matters, and the practical next steps. If your situation needs tailored advice, contact the RNA Law team.
Q: What changed, and why is it not “business as usual”?A: ANVISA reframes expectations in competition & regulatory strategy. Analysis of Decree #12,651/2025: What the Decree Effectively Addresses Institutional Structure and Ethical Governance . Q: Which parts of the business will feel this first?A: Typically: the operational teams executing the rule (approvals/filings, product/service design, contracting) and the lines that must prove compliance under scrutiny. . Q: What is the main enforcement / dispute risk executives should manage?A: Misalignment between policy and execution plus weak documentation are the usual failure modes. Decree #12,651/2025 establishes the National System of Ethics in Research Involving Human Subjects (SINEP) and seeks to strike a balance between ethical protection for research participants and regulatory efficiency, representing the most. Set ownership, define. Q: What should we do in the next 30–90 days?A: Commission a targeted gap assessment; prioritize high-impact processes; update playbooks and controls; and issue an executive memo with options, costs, timing, and residual risk. Align Legal, Compliance, Operations, and business owners on one plan.